Calculate Temperature Change Rate Thermodynamics is the field of physical science worried about temperature, heat and, ultimately, energy transfers. Although the laws of thermodynamics can be somewhat precarious to keep, the primary law of thermodynamics is a straightforward relationship between the work done, heat added, and the change in internal energy of a substance.
If you have to calculate a Temperature Change Rate, it’s either a basic course of subtracting the old temperature from the upgraded one, or it may include the main law, the amount of energy added as heat, and the specific heat capacity of the substance being referred to.
Sorry for the basic inquiry however I have gone over the formula ºC.min-1 in a paper which I accept is the formula for temperature change during one moment. The thing is that when I go to perceive how the authors utilized it, they have greater temperature change values than the ones a basic utilization of the formula suggests in my interpretation (for instance, T1=27º; T2= 28º; T1 and T2 is brief span so Temperature Change Rate is 1ºC).
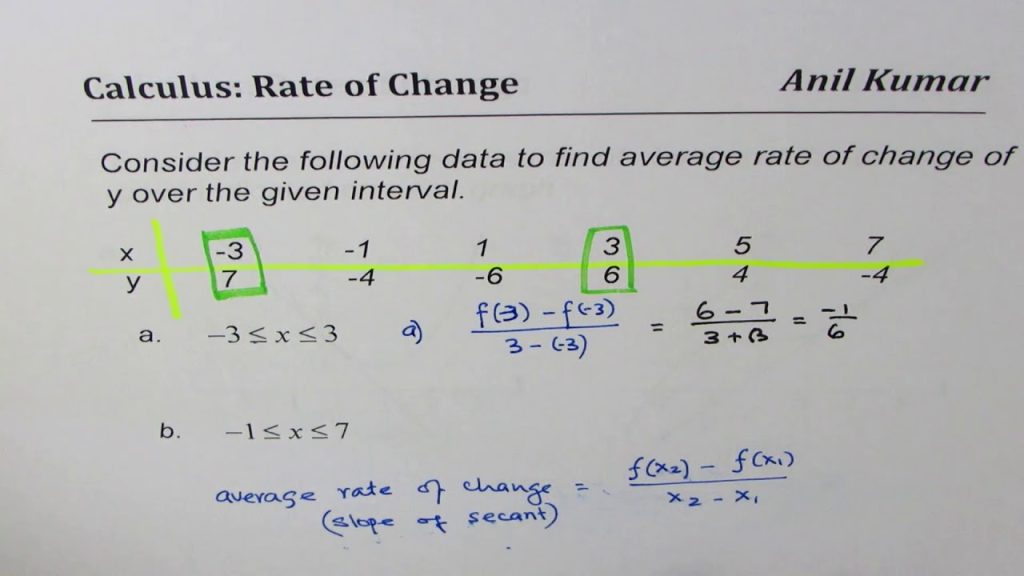
Calculating Temperature Change Rate
Its a well known fact that the Temperature Change Rate high up in the mountains will be lower than that in the valleys. Have you at any point set off on a warm summer climb to track down the temps at the peak in the 50’s or more terrible?
Avoid these surprises by knowing how to calculate the temperature misfortune as you climb. Temperature Calculator Here are the means in question, and a straightforward equation to reference.
Presently, before we continue, these are straightforward approximations. They’re not exact, they’re not horribly scientific, and they’re not meant to be utilized in such a way. Also, if you do the math utilizing Celcius versus Fahrenheit, you will get somewhat different values. Again, these are both simplified calculations intended to be done on the fly, they’re not exact. That being said, how about we go on.
Look up the weather forecast
Clearly, you really want a base to go on. Look up the local area forecast, and see what the high, and low temperatures will be.
Determine the elevation of reference for the forecast.
All weather forecasts are referenced to a particular elevation. Utilizing the National Weather Help site you can a detailed forecast, and they’ll list the elevation of reference on the page. If that information isn’t available, it’s usually the same elevation as your official city elevation. Here, we’re at 2,000 feet, and our forecast are all for 2,000 feet.
Determine your peak elevation
Presently you want to know how high up you will climb or slip. Reference your topo map, or find these details on the web. A speedy Google with “Mountain (Name) elevation” will normally get you what you really want.
Do the math
You will lose an average 3.5 degrees Fahrenheit for each 1000 feet of elevation you gain. You can also use about 1.2 degrees Celsius per ever 1000 feet, or about 1 degree Celsius for each 100 meters (source, NFW who showed me my grammatical mistake on the metric change in the remarks). Certain individuals utilize 9.8 degrees Celsius per 1000 meters).
Factors that affect your actual temperature
A few factors change the actual value of these calculations. Overcast cover will trap in more heat, where a clear sky will decrease the temperature somewhat faster. Cold fronts and air streams may also have an impact, as well as local evaporative cooling. These factors are too various to account for, so the equation is planned as a general scenario calculation (after adjusting).
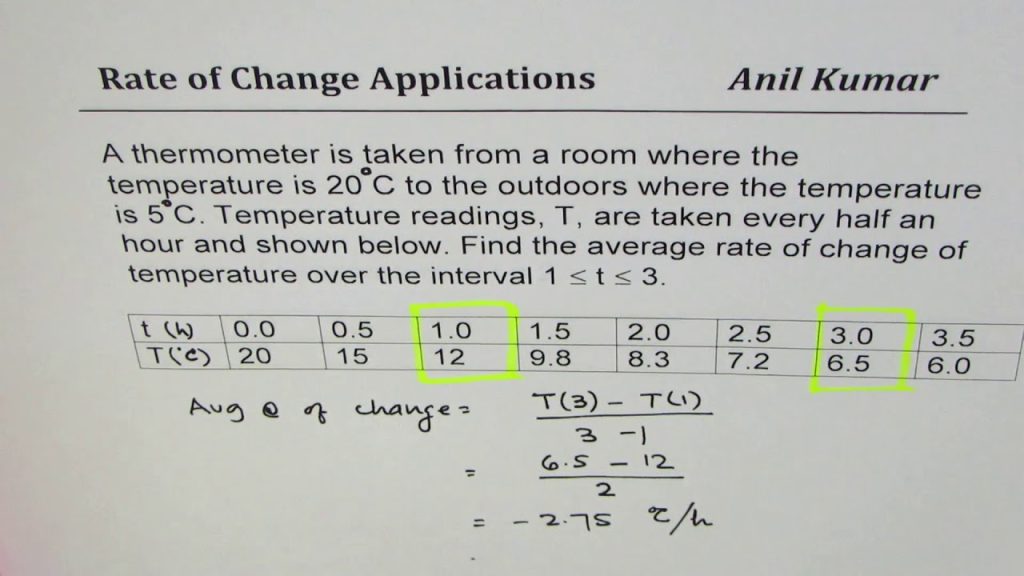
How do you calculate the rate of temperature change with a given graph?
Basic! To start with, it is assumed you have a plot of temperature (T) versus time (t). If you want the rate of change of temperature at a particular time (you have to specify a particular time since the rate of temperature may be changing with time), all you want is the slant of the line tangent to the T versus t bend at the chose time.
How you draw the tangent line is up to you. Reduce CPU Temperatures in 2022 If you have a functional relationship among T and t, then the derivative DT/dt provides you with the rate of change of T (slant of the tangent line). You can also get an estimate of the derivative numerically utilizing deeply grounded methods.
We generally talk about this issue utilizing the primary law of thermodynamics which is the conservation of energy. If you have mass stream and a change a Temperature Change Rate then you are a going through a cycle between two states. In a shut framework we always have conservation of mass so the mass coming into the framework equals the mass leaving. It may had different streams or a different chemical creation however mass is as yet constant (note I have overlooked nuclear impacts so this does not apply to nuclear reactions).
Is there a way to calculate temperature change and pressure change if volume is decreased?
If it does, it would be immeasurably small. Your pee will be warm and so it will carry away a portion of the body’s heat. Be that as it may, heat is not the same as temperature. Your body will have an exceptionally large thermal mass, so whatever heat is carried away in the couple of ounces of liquid lost in the pee won’t change your internal heat level a lot.
The Joined Gas Law or General Gas Equation is obtained by consolidating Boyle’s Law, Charles’ Law, and Gay-Lussac’s Law. It shows the relationship between the pressure, volume, and temperature for a decent mass (quantity) of gas.
The van Der Waal’s Equation (or van Der Waal’s Equation of state) was first presented by van Der Waals in 1877 and is based on plausible reasons that real gases do not keep the ideal gas law. The ideal gas law treats gas atoms as point particles that do not interact besides in elastic collisions. In other words, they do not take up any space, and are not attracted or repulsed by other gas particles.
















Leave a Reply