If you have a substance and you need to rapidly Calculate Weight Percent From Molarity over completely to molarity, our tool does as such in three straightforward advances. Sit back and relax on the off chance that you don’t have a clue about the molar mass of a given arrangement – we’ve given you a rundown of the most famous ones. Recall that our calculator works the two different ways – you don’t have to enter your qualities from top to bottom.
Focus addresses how much the compound broke up in the arrangement. Weight Convertors is the quantity of moles of a substance in 1 liter of the arrangement. One more unit of the focus, weight percentage, alludes to the proportion of the mass of the solute (a broke up substance) to the mass of the arrangement. Changing over between focuses is oftentimes expected for different issues in science.
In going before segments, we zeroed in on the sythesis of substances: tests of issue that contain just a single sort of component or compound. However, combinations — tests of issue containing at least two substances genuinely joined — are more usually experienced in nature than are unadulterated Calculate Weight Percent From Molarity. Like an unadulterated substance, the general piece of a combination assumes a significant part in deciding its properties.
Utilize the believer percentage fixation to molarity calculator?

Working out molarity with our calculator will presumably take you less time than it did to peruse the title of this part
Follow the means beneath to rapidly get your outcome:
Do you know the molar mass of your substance?
In the event that you don’t, attempt to find it on our rundown of the most well known substances utilized in science. Kindly pick the custom choice and enter the known worth assuming that you know it.
Molar mass is the mass of 1 mole of a substance, given in g/mol. 1 mol comprises of precisely 6.02214076 × 10²³ particles.
Enter the thickness of your answer.
Make sure to twofold really take a look at the states of the response, focus and the weakening of your answer!
Enter the Calculate Weight Percent From Molarity of your answer or the molarity of your answer.
The molarity, A.K.A. the molar focus, portrays the quantity of moles in a given volume of arrangement. We as a rule use units like 1 mol/L (moles per liter) = 1 mol/dm³ (moles per cubic decimetre) = 1 M (molar).
Your outcomes have been calculated!
You can likewise check assuming your synthetic responses are occurring in standard circumstances.
How would I switch molarity over completely to percentage focus?
Here is the condition we use to switch the percentage fixation over completely to molarity:
- Molarity = (Percentage focus × Thickness )/(Molar mass × 100)
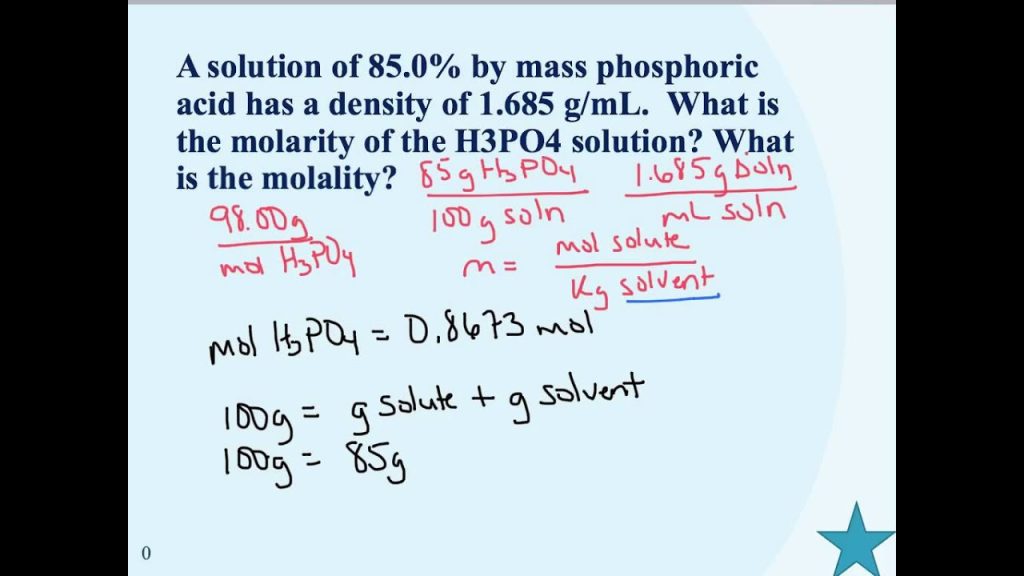
The units expected for this computation are:
- Molarity – > mol/dm³ = M = mol/L;
- Percentage focus – > %;
- Thickness – > g/L = g/dm³; and
- Molar mass – > g/mol.
Duplicate the molar mass of the compound by the Calculate Weight Percent From Molarity how much the broke up substance in one liter of the arrangement. For instance, 0.5 M of KCl arrangement contains 74.5 x 0.5 = 37.25 g of the salt.
Increase the thickness of the arrangement by 1,000 ml (1 liter) to calculate the mass of the 1L of the arrangement. For instance, assuming the thickness of 0.5 M KCl arrangement is 1.1 g/ml, the weight of 1 liter of the arrangement is 1.1 x 1,000 = 1,100 g.



















Leave a Reply